ATOM AND MOLECULE
The Nobel Prize in Physics for 2023 has gone to three scientists whose work made it easier to observe electrons, and which has potential applications in the field of diagnosing diseases and developing electronic gadgets.
Anne L’Huillier, Pierre Agostini, and Ferenc Krausz have been awarded for their experiments “which have given humanity new tools for exploring the world of electrons inside atoms and molecules. They have demonstrated a way to create extremely short pulses of light that can be used to measure the rapid processes in which electrons move or change energy,” said the Nobel Prizes website
2. What is an Atom?.

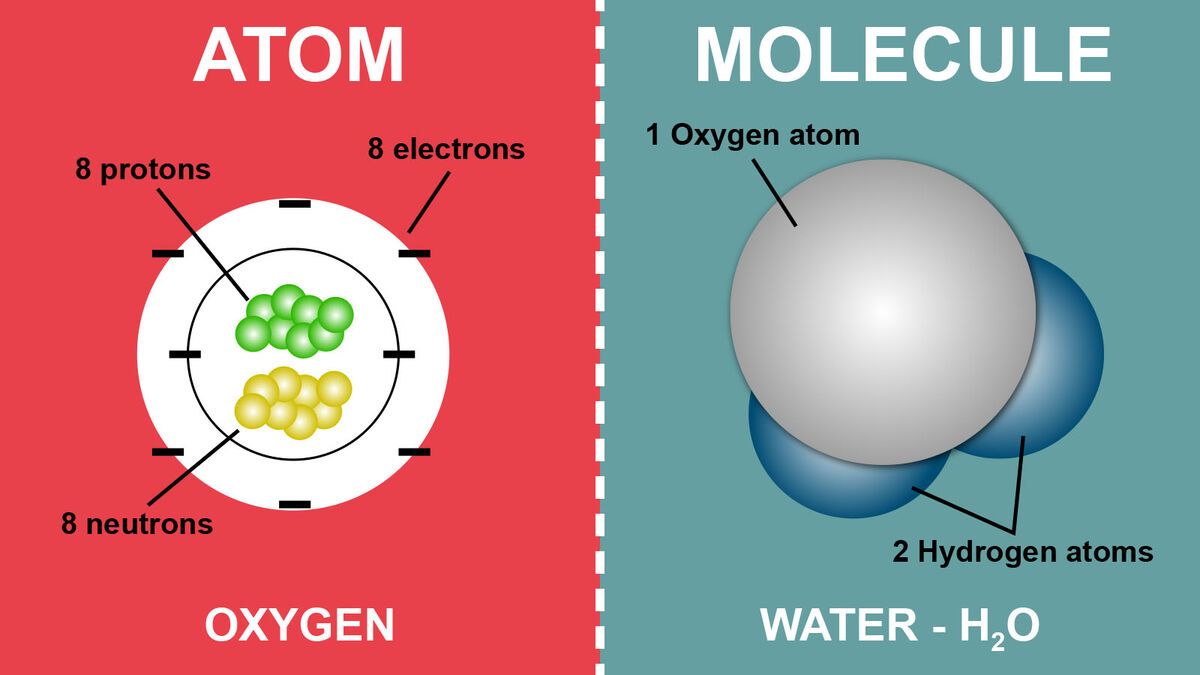
- An atom is the basic unit of matter and the fundamental building block of chemical elements. Atoms are incredibly small and make up everything in the universe, including solids, liquids, gases, and even living organisms. They are the smallest entities that retain the properties of a chemical element.
- The size of an atom can be somewhat challenging to define precisely because atoms do not have well-defined boundaries like macroscopic objects. Instead, they have a finite volume where there is a high probability of finding electrons.
- The size of an atom is typically described in terms of its atomic radius, which represents the average distance from the nucleus to the outermost electron cloud
- The size of atoms can vary depending on the specific element and its electron configuration. However, a rough estimate of the typical size of an atom is on the order of 0.1 to 0.2 nanometers (nm), or about 1 to 2 angstroms (Å).
- For example, the atomic radius of a hydrogen atom is approximately 0.053 nm (0.53 Å), while the atomic radius of a helium atom is about 0.049 nm (0.49 Å).
Key characteristics and components of an atom include:
-
Nucleus: At the center of an atom is the nucleus, which contains two types of subatomic particles:
- Protons: Positively charged particles found in the nucleus.
- Neutrons: Neutrally charged (no charge) particles also located in the nucleus.
-
Electrons: Electrons are negatively charged particles that orbit the nucleus in electron shells or energy levels. These electrons move rapidly around the nucleus in specific energy levels or orbits.
-
Subatomic Structure: Atoms consist of a specific number of protons, neutrons, and electrons. The number of protons defines the element and gives it its unique chemical properties. The sum of protons and neutrons in the nucleus is referred to as the atomic mass or mass number.
-
Neutral Charge: Atoms are electrically neutral because the number of protons in the nucleus is equal to the number of electrons in orbit. The positive charge of protons cancels out the negative charge of electrons, resulting in a net charge of zero.
- Isotopes: Some elements can have variations in the number of neutrons, resulting in different atomic isotopes of the same element. Isotopes of an element have the same number of protons but different numbers of neutrons.
Here are some key points about molecules:
-
Composition: Molecules are composed of atoms. The atoms in a molecule are held together by chemical bonds, which can be covalent (sharing of electrons) or ionic (transfer of electrons).
-
Chemical Formula: Molecules are represented by chemical formulas that indicate the types and numbers of atoms in the molecule. For example, the chemical formula for water (H2O) tells us that one molecule of water consists of two hydrogen atoms (H) bonded to one oxygen atom (O).
-
Stable Structures: Molecules represent stable and distinct structures. When atoms come together to form molecules, they achieve a more stable and lower-energy state than when they are isolated.
-
Properties: The properties of a substance, including its physical and chemical properties, are determined by the types of molecules it contains and how those molecules are arranged and interact with each other.
-
Size: Molecules can vary in size from small and simple, like the diatomic molecules mentioned earlier, to very large and complex, such as the DNA molecule in living organisms.
-
Bonding: The way atoms are bonded together within a molecule can have a significant impact on the molecule's properties. For example, covalent bonds involve the sharing of electrons and result in molecules with distinct shapes and properties, while ionic bonds involve the transfer of electrons between atoms.
-
Reactivity: Molecules can participate in chemical reactions. During a chemical reaction, molecules can break apart and form new molecules through the rearrangement of atoms and bonds.
- An atom, a tiny unit into which matter can be divided, is composed of a nucleus of protons and neutrons, and electrons that travel around this nucleus. Electrons move so fast that it is impossible to observe them in real time.
- The work of L’Huillier, Agostini, and Krausz has brought humanity closer to observing and studying the movement of electrons, by producing pulses of light that last only attoseconds, which is 1×10−18 of a second.
- Roughly, this can be compared to a high-shutter-speed camera. If a normal camera is used to capture a moving train, the image will be blurred. But a high shutter-speed camera can freeze motion and capture a clear image of the train
- All three worked in the field for years. According to the Nobel website, back in 1987, L’Huillier discovered that when a laser light wave was passed through a noble gas, it interacted with the atoms, giving some electrons extra energy that was then emitted as light. She continued to work on this.
- These flashes of light made it possible to provide images of processes inside atoms.
- We can now open the door to the world of electrons. Attosecond physics gives us the opportunity to understand mechanisms that are governed by electrons. The next step will be utilising them
- One possible application is to study molecular-level changes in blood, to identify diseases
- A better understanding of how electrons move and transmit energy can also help in creating more efficient electronic gadgets.




